Imagine an EYE OPENING Lift with a Daily Drop
Upneeq®
(Oxymetazoline Hydrochloride ophthalmic solution), 0.1%
Upneeq®
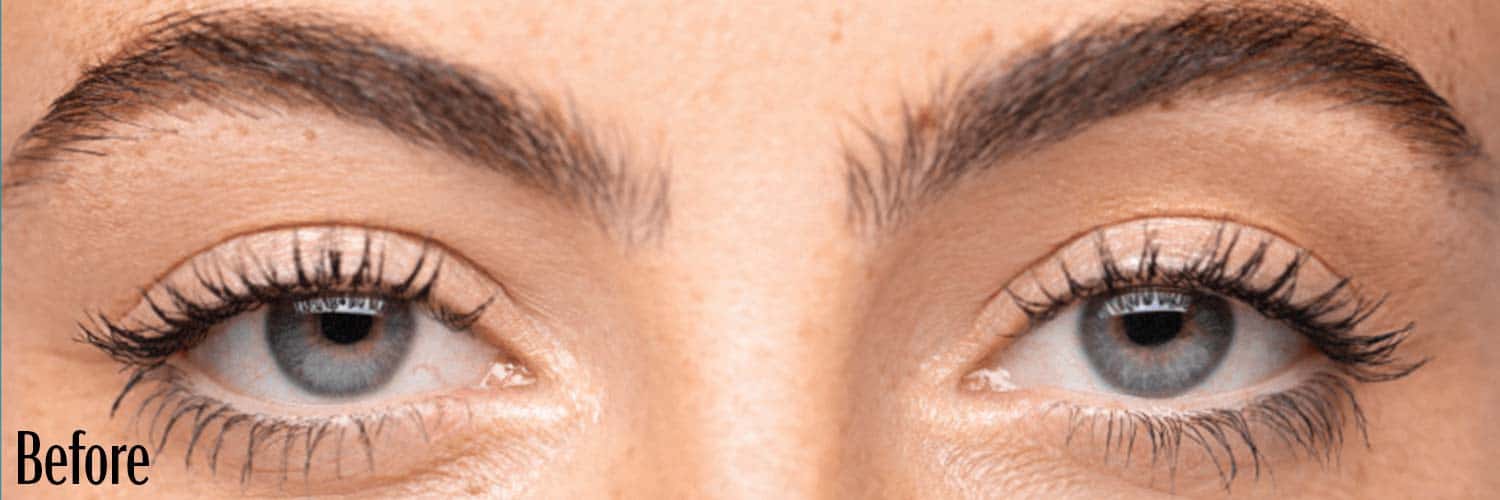
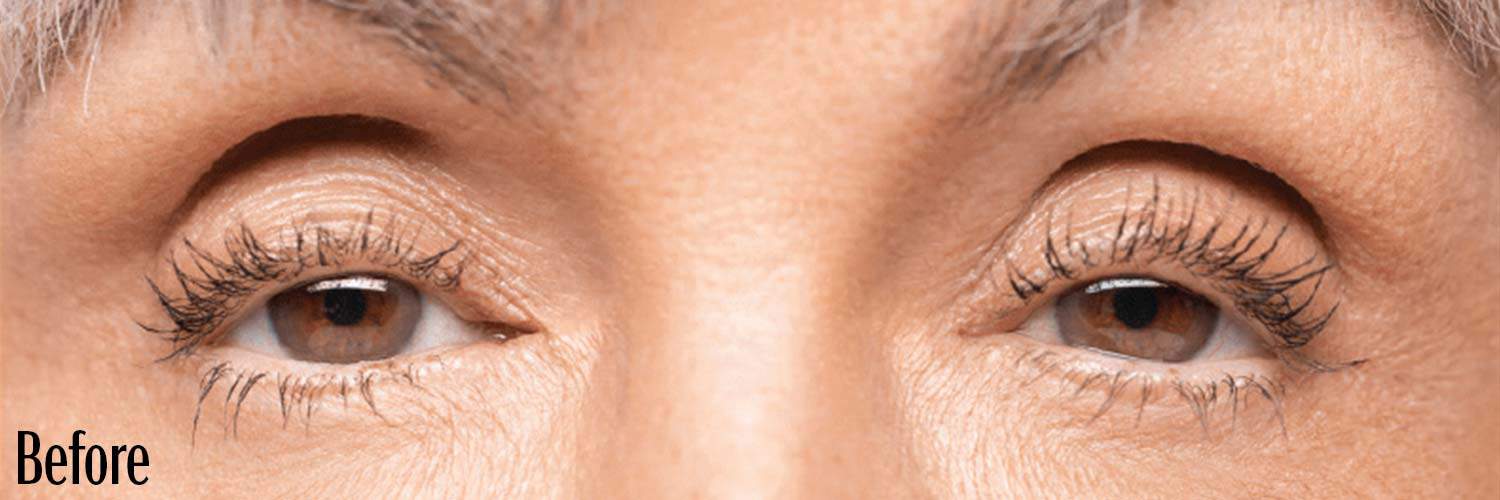
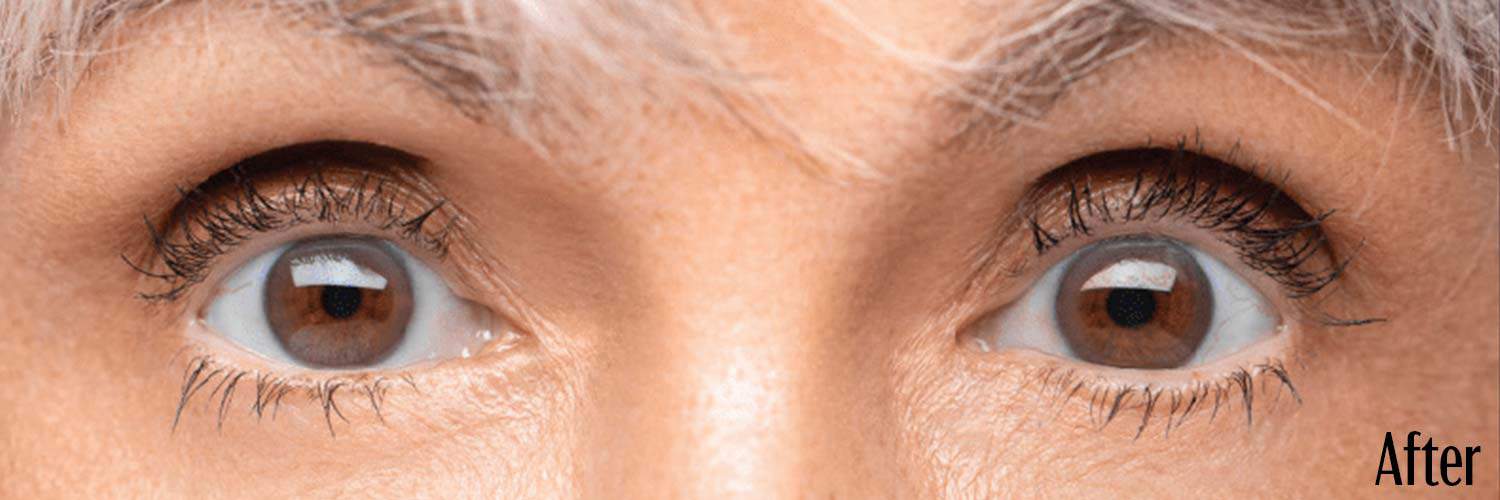
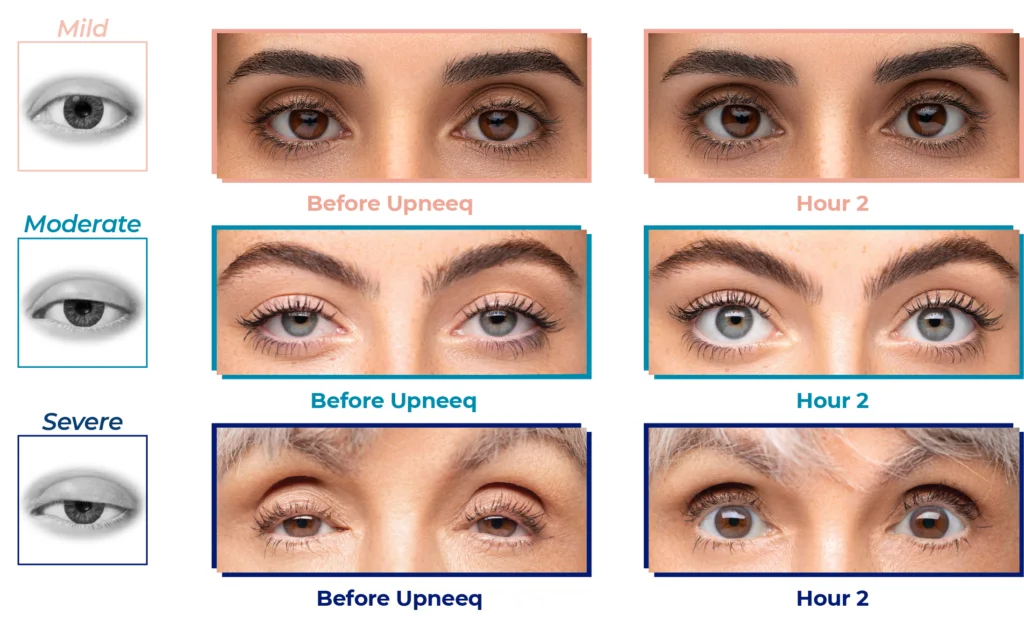
Before and After
Why do my eyelids look low?
If your eyes look “tired” or “sleepy,” you may have a condition called acquired blepharoptosis (also known as acquired ptosis or low-lying lids).
Impact of Low-Lying Lids
Low-lying lids can affect the way you look and see things.
Upneeq®
Upneeq is the only FDA-approved prescription eyedrop for acquired ptosis (low-lying lids) that lifts your upper eyelids to open your eyes
Upneeq is the only FDA-approved prescription eyedrop for acquired ptosis (low-lying lids) that lifts your upper eyelids to open your eyes
UPNEEQ®
(oxymetazoline hydrochloride ophthalmic solution), 0.1%
Low-lying lids may be caused by acquired blepharoptosis.
If you see signs of low-lying lids, talk to your RSVP Esthetician about getting UPLIFTED with Upneeq!1
PRODUCT INFORMATION
Warnings and Precautions
- Ptosis may be associated with neurologic or orbital diseases such as stroke and/or cerebral aneurysm, Horner syndrome, myasthenia gravis, external ophthalmoplegia, orbital infection and orbital masses. Consideration should be given to these conditions in the presence of ptosis with decreased levator muscle function and/or other neurologic signs.
- Alpha-adrenergic agonists as a class may impact blood pressure. Advise UPNEEQ patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension or hypotension to seek medical care if their condition worsens.
- Use UPNEEQ with caution in patients with cerebral or coronary insufficiency or Sjögren’s syndrome. Advise patients to seek medical care if signs and symptoms of potentiation of vascular insufficiency develop.
- UPNEEQ may increase the risk of angle closure glaucoma in patients with untreated narrow-angle glaucoma. Advise patients to seek immediate medical care if signs and symptoms of acute narrow-angle glaucoma develop.
- Patients should not touch the tip of the single patient-use container to their eye or to any surface, in order to avoid eye injury or contamination of the solution.
Adverse Reactions
Adverse reactions that occurred in 1-5% of subjects treated with UPNEEQ were punctate keratitis, conjunctival hyperemia, dry eye, blurred vision, instillation site pain, eye irritation and headache.
Drug Interactions
- Alpha-adrenergic agonists, as a class, may impact blood pressure. Caution in using drugs such as beta blockers, anti-hypertensives, and/or cardiac glycosides is advised. Caution should also be exercised in patients receiving alpha adrenergic receptor antagonists such as in the treatment of cardiovascular disease, or benign prostatic hypertrophy.
- Caution is advised in patients taking monoamine oxidase inhibitors which can affect the metabolism and uptake of circulating amines.
Report Adverse Reactions
To report SUSPECTED ADVERSE REACTIONS or product complaints, contact RVL Pharmaceuticals at 1-877-482-3788. You may also report SUSPECTED ADVERSE REACTIONS to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
References: 1. Upneeq® (oxymetazoline hydrochloride ophthalmic solution) 0.1%. [Prescribing Information]. RVL Pharmaceuticals, Inc. 2020. 2. Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003;27(3):193-204. 3. Richards HS, Jenkinson E, Rumsey N,et al. The psychological well- being and appearance concerns of patients presenting with ptosis. Eye. 2014; 28(3):296-302. 4. Data on file, RVL Pharmaceuticals, Inc
Learn More
Request A Consultation
RSVP MEDSPA & COSMETIC EYE INSTITUTE
13300 Metcalf Ave.
Overland Park, KS 66213
Hours
Monday – Thursday: 9:00 AM – 5:00 PM
Friday: 9:00 AM – 4:00 PM
Saturday: 9:00 AM – 4:00 PM
Sunday: CLOSED