LATISSE®
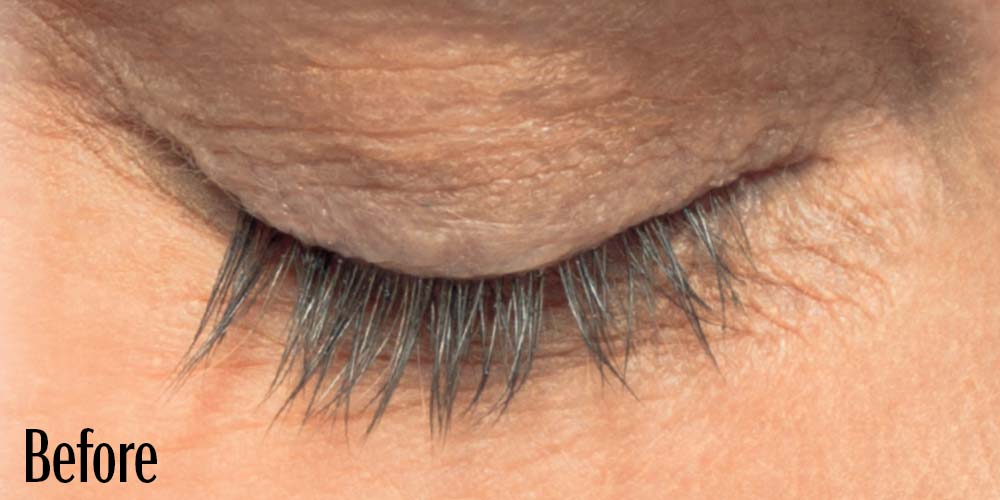
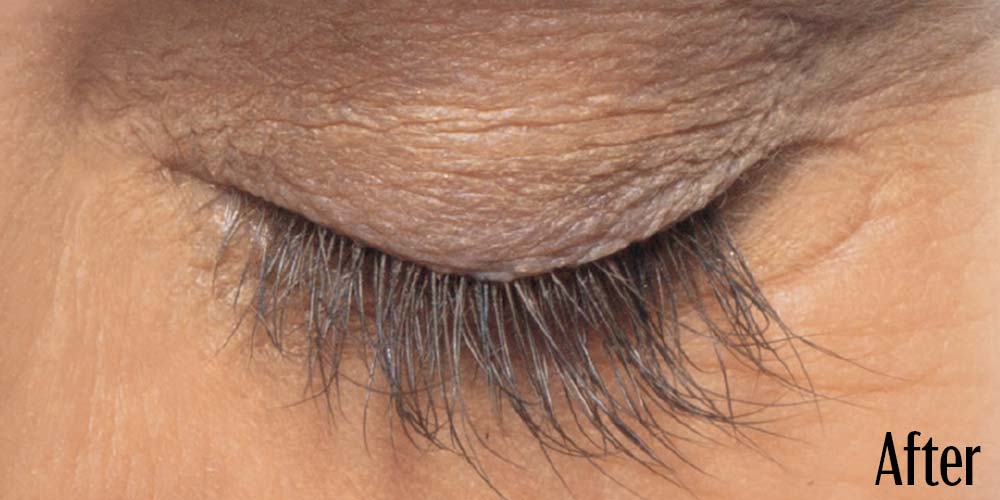
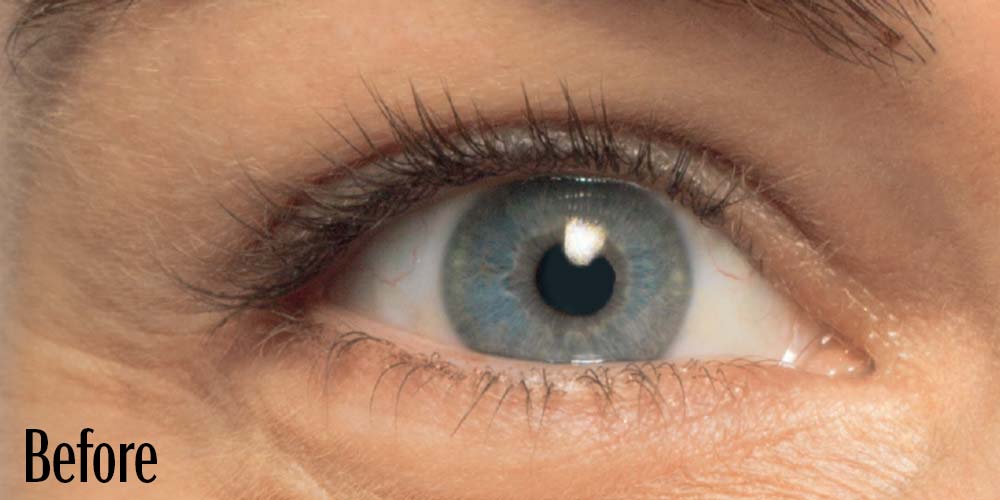
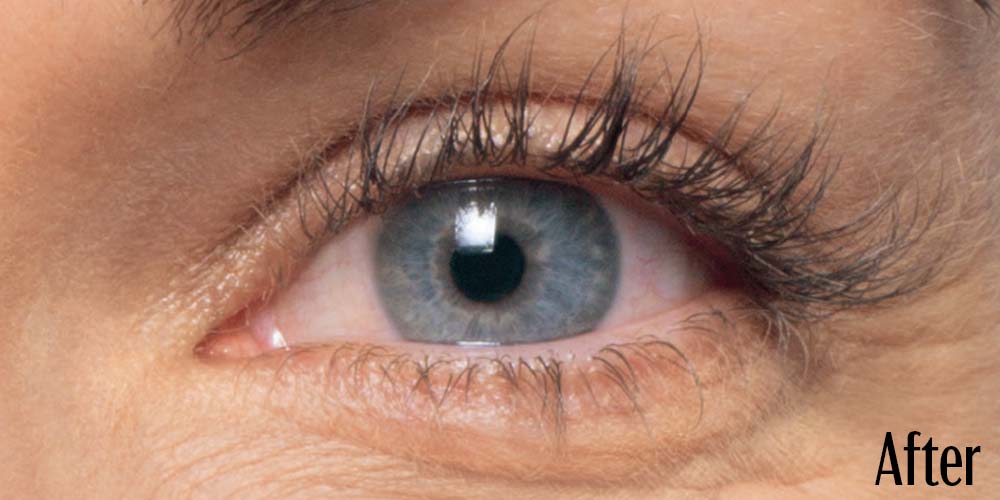
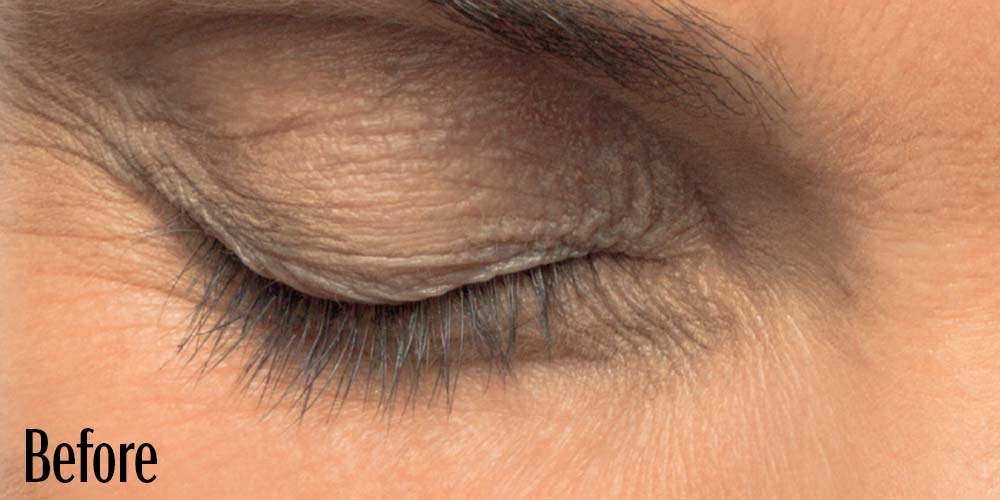
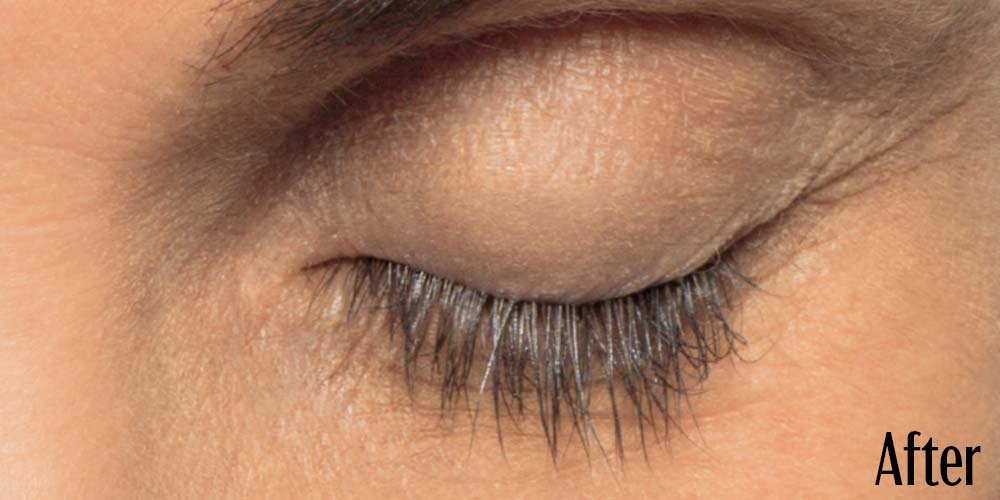
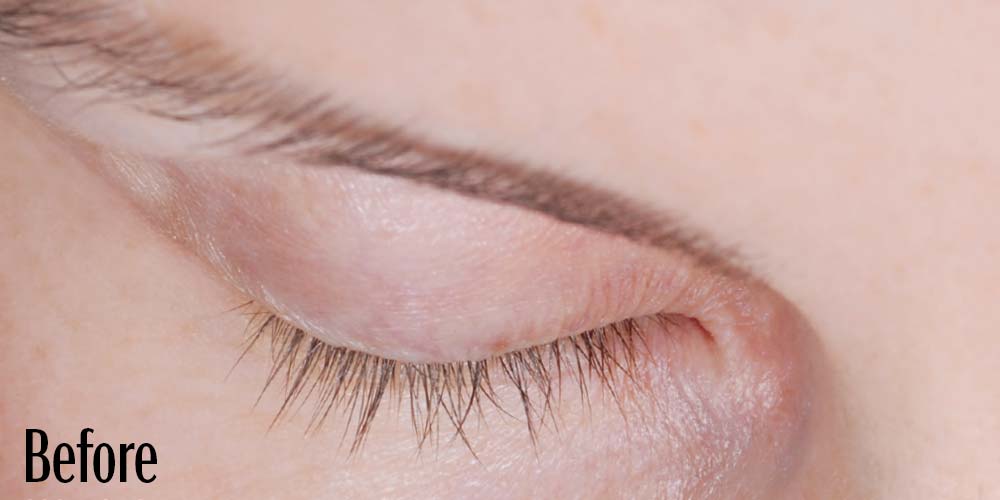
Before and After
LATISSE® (BIMATOPROST OPHTHALMIC SOLUTION) 0.03%
Latisse is clinically proven to deliver lashes that are longer, fuller, and darker in just 16 weeks. If you have inadequate lashes, contact us to see if LATISSE® solution is right for you.
REQUEST A CONSULTATION
WHAT HAPPENS IF I STOP USING LATISSE®?
Latisse treats inadequate lashes by prolonging the active eyelash growth phase. If you stop using LATISSE®, your eyelashes are expected to return to their previous appearance over several weeks to months. Any eyelid skin darkening is expected to reverse after several weeks to months. Any darkening of the colored part of the eye known as the iris is NOT expected to reverse and is likely permanent.
REQUEST A CONSULTATIONHOW DO I USE LATISSE®?
The recommended dosage is one application nightly to the skin of the upper eyelid margin at the base of the eyelashes only. Once nightly, start by ensuring your face is clean, and makeup and contact lenses are removed. Remove an applicator from its tray. Then, holding the sterile applicator horizontally, place one drop of LATISSE® on the area of the applicator closest to the tip but not on the tip. Then immediately draw the applicator carefully across the skin of the upper eyelid margin at the base of the eyelashes (where the eyelashes meet the skin) going from the inner part of your lash line to the outer part. Blot any excess solution beyond the eyelid margin. Dispose of the applicator after one use. Repeat for the opposite upper eyelid margin using a new sterile applicator. This helps minimize any potential for contamination from one eyelid to another.
LATISSE®
(bimatoprost ophthalmic solution) 0.03%
Indicated to treat hypotrichosis of the eyelashes by increasing their growth, including length, thickness, and darkness. Latisse is a prescription product and must be prescribed by a licensed healthcare provider.
IMPORTANT SAFETY INFORMATION (BELOW)
- CONTRAINDICATIONS
- ADVERSE REACTIONS

Prescription treatments are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use LATISSE® solution for a condition for which it was not prescribed. Do not give LATISSE® to other people. It may not be appropriate for them to use. This leaflet summarizes the most important information about LATISSE® solution. If you would like more information, talk with your physician. You can also call Allergan’s product information department at 1-800- 678-1605. What are the ingredients in LATISSE®?
Active ingredient: bimatoprost
Inactive ingredients: benzalkonium chloride; sodium chloride; sodium phosphate, dibasic; citric acid; and purified water. Sodium hydroxide and/or hydrochloric acid may be added to adjust pH. The pH during its shelf life ranges from 6.8 – 7.8.
How does latisse work?
Request a Consultation
Are you ready to take the first step to achieving your goals? Request a consultation today! Or, if you’re ready to book your appointment, click the button below and we will reach out to you.
Book appointment